QOMS Audit registries
To date, QOMS has been operating audits across 6 OMFS subspecialties:
- Oral & dentoalveolar surgery
- Trauma
- Oncology
- Reconstruction
- Non-melanoma skin cancers
- Orthognathic surgery
The current data collection cycle officially started in July 2021 in England, Wales and Scotland and is due to finish in 2024. An Inaugural report was published in 2023 covering the first year of data collection (2021-2022). This report mainly focused on ascertaining that data across the registries would be of high quality to produce comparative results by the end of the current phase in 2024. Some preliminary comparative data was included in the report for Oncology & Reconstruction and Non-Melanoma Skin Cancers.
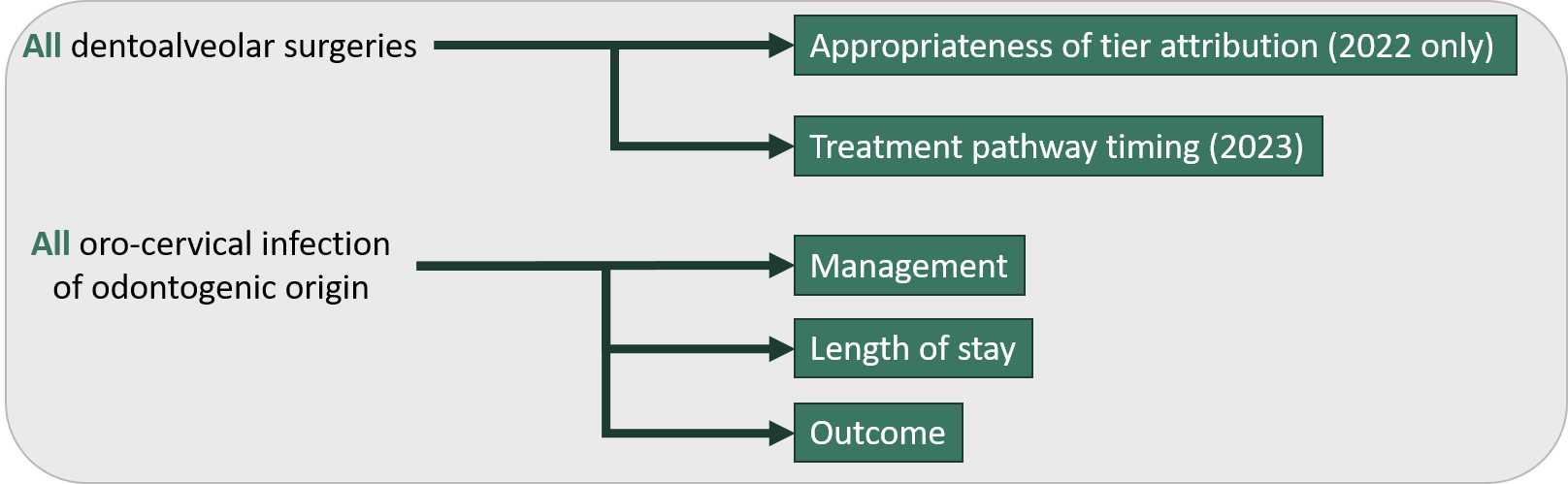
Oral & dentoalveolar surgery
Oral and dentoalveolar (ODA) surgery activity is assessed using 2 registries. The low-risk / high-volume nature of the ODA work means that the metrics of interests are mainly process measures.
Figure 1 - Oral & dentoalveolar surgery
Audit Lead: Mr Geoff Chiu
Data collection: snapshot
- 2022 Period: 03/01/2022 to 04/03/2022 (BAOMS-funded units)
- 2023 Period: 04/09/2023 for as long as necessary to obtain 200 consecutive records
Clinical Report Forms / Questionnaires
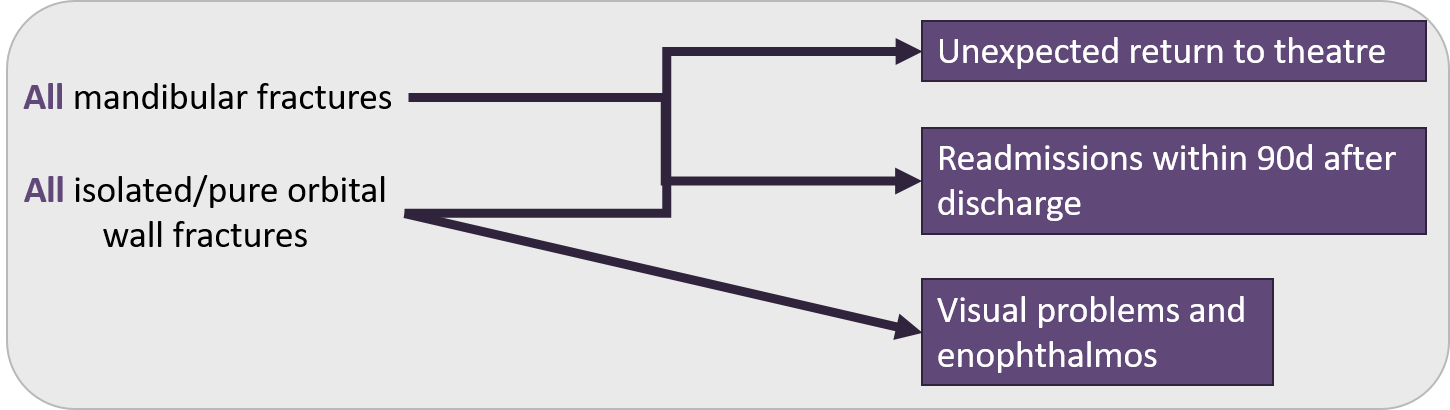
Trauma
Figure 2 - Trauma
Audit Lead: Mr Geoff Chiu
Data collection: continuous
Start of data collection: July 2021
Clinical Report Forms / Questionnaires
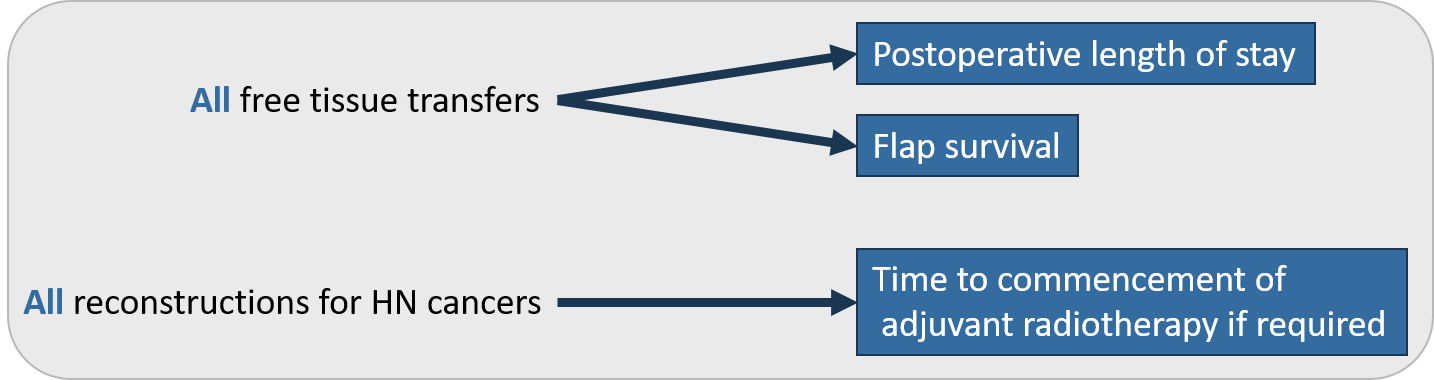
Oncology & Reconstruction
As a large part of the oncology and reconstruction activity overlaps, oncology and reconstruction are being assessed within one registry.
Figure 3 - Oncology
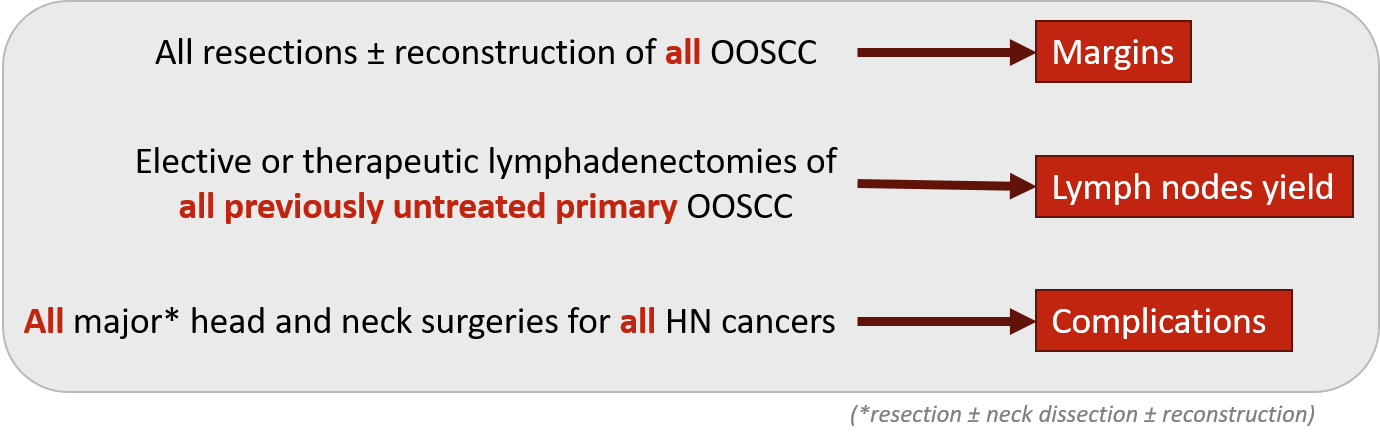
Figure 4 – Reconstruction
Audit Lead: Mr Panayiotis Kyzas
Data collection: continuous
Start of data collection: July 2021
Clinical Report Forms / Questionnaires
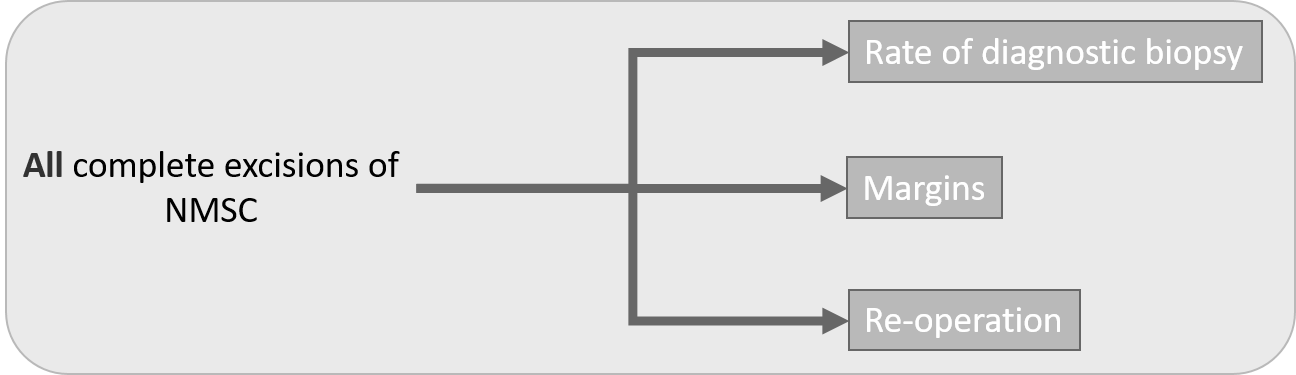
Non-melanoma skin cancers
The Non-Melanoma Skin Cancer (NMSC) activity record all NMSC registry records all NMSC activity but its reporting focuses on SCCs and BCCs.
Figure 5 - Non-melanoma skin cancers
Audit Lead: Mr Panayiotis Kyzas
Data collection: snapshot
- 2021 Period: 13/09/2021 to 13/11/2021 (BAOMS-funded units)
- 2022 Period: (a) 16/05/2022 to 15/07/2022 and (b) 10/10/2022 to 30/11/2022 (BAOMS-funded units)
- 2023 Period: 02/05/2023 to 30/06/2023 or for as long as necessary to obtain 200 consecutive records
Clinical Report Forms / Questionnaires
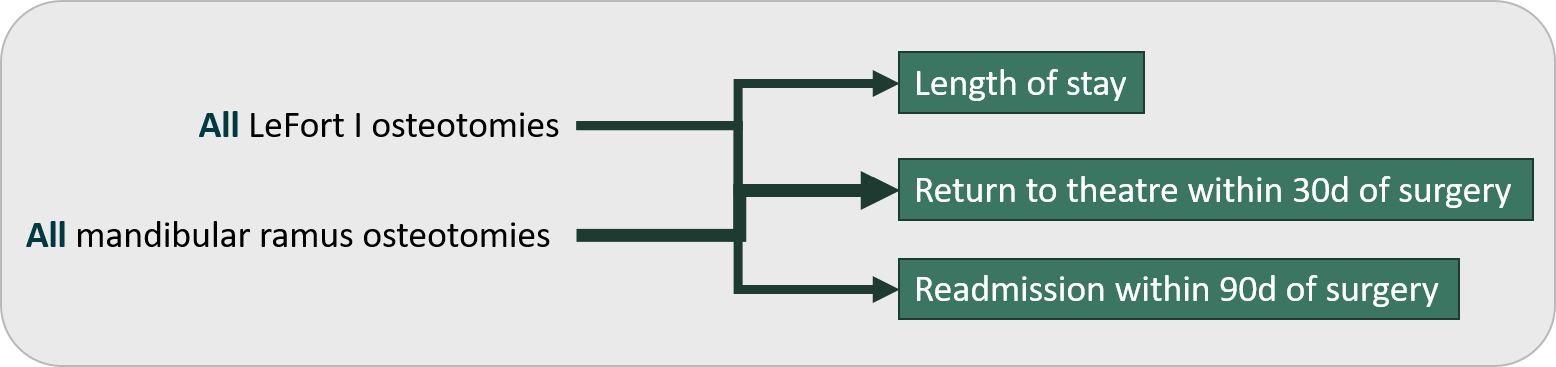
Orthognathic surgery
The Orthognathic registry assess quality of care across all orthognathic surgery activity but the reporting will mainly focus on LeFort I and mandibular ramus osteotomies.
Figure 6 – Orthognathic surgery
Audit Leads: Professor Ashraf Ayoub, Mr Moorthy Halsnad, Ms Christine Lwin, and Audit Deputy Lead: Mr Alexander Hills
Data collection: continuous
Start of data collection: August 2021
Clinical Report Forms / Questionnaires
The orthognathic component of QOMS will also collect patient reported outcome (PRO). A new PRO tool to replace the existing ones has been selected in collaboration with the British Orthodontic Society (BOS). To find out more, see the Condition- and procedure-specific Registries page.
Contact the project manager to discuss other methods of data collection (e.g. spreadsheets etc).
Timeline
2018-2019: Project development
- Consultation with BAOMS SSIG Leads and Deputy Leads about what shape the initiative should take, which OMFS subspecialties would be involved and which metrics to measure
- Development of QOMS Protocol, questionnaires
- Selection of a IT solution for data collection and storage
- December 2019 - March 2020: launch of the QOMS first pilot in 6 OMFS units in England to test the online system, the registries and data collection processes locally.
2020-2021: COVID
- The initial QOMS pilot was cut short by a few weeks by the start of the COVID pandemic and QOMS activity was put on hold during that time.
- The QOMS Team ran several snapshot audits looking at the impact of the pandemic on the provision of care for OMFS trauma and cervico-facial infections. Find out more here.
- In parallel, the QOMS registries were revised based on the feedback from the pilot - for more details, read Ho et al. (2021) BJOMS.
- The content, structure and flow of the registries needed to be improved. This was undertaken by the newly appointed QOMS Audit Groups and the SSIGs.
- Consenting patients for QOMS had been identified as a hurdle in the pilot. It was difficult to implement locally and increased clinicians’ workload. Where possible, it was decided to apply to devolved authorities to gain approval to collect patient (identifiable) information without consent.
- Surgeon-led data collection was not sustainable.
2021-2024: QOMS Second Pilot (on-going)
- To support the project and the process, the Association decided to fund 10 OMFS units to employ a part-time data coordinator to effectively support high-quality and comprehensive data collection with the help of the hospital Clinical Lead. This pump-priming grant would be for 3 years and would a pilot to demonstrate the value of encouraging NHS Trusts and Health Boards to support this process in the long term.
- Application for this funding opened to every OMFS units nationwide at the end of 2020. Unit selection was based on a competitive process: track record in engagement with quality improvement, national audit and academic outcomes. Successful applications required the committed engagement from all local OMF surgeons and the hospitals’ medical directors.
- The appointed data coordinator will work closely with the local QOMS clinical lead and other surgeons to collect and update data for the project. Behind that 3-year initial funding, BAOMS hope:
- To have demonstrated the importance and value of the QOMS programme and
- That OMFS departments will be able to secure local funding from their NHS Trusts and Health Boards to continue data collection or to start taking part to the QOMS.
- The application form and a job description to help hospitals recruit their data co-ordinator have been prepared and approved by BAOMS Council. Job description for data-co-ordinator is available here.
2021-2024: QOMS first data collection cycle (on-going)
- It was decided that QOMS would be rolled out nationally in the summer of 2021 to every OMFS unit and/or surgeon in the UK. This was effectively the initiation of the definitive QOMS data collection process.
- The QOMS Team applied to the Confidentiality Advisory Group (CAG) for section 251 support for England and Wales and to the Public Benefit and Privacy Panel for Health and Social Care (PBPP) for Scotland. Approvals were obtained in 2020 and 2022 respectively.
- Data collection officially opened in July 2021. BOAMS-funded departments could join as soon as they had successfully hired a data coordinator and obtained Trust/ Health Board approval for QOMS.
- At the end of the 1st year of data collection, the QOMS Team censored the data and produced the QOMS Inaugural report.
- The 2nd QOMS report should be published at the end of 2024 and include the whole summer 2021 to summer 2024 period (censure date TBC).
Information Governance considerations for QOMS
- QOMS is not considered research but audit / service evaluation. Therefore it does not require ethical approval. Secondary research is however possible and not incompatible, but appropriate ethical approval should be obtained.
- QOMS has been granted approval to collect patient information without consent in England and Wales (CAG s251) and Scotland (PBPP). Approval from Hospital / Trust / Health Board still needs to be granted before the project can start at any location.
- Despite being able to collect patient information without consent, participating hospitals still have the duty to inform patients. The QOMS Team has developed with a patient group posters to be displayed in clinics and waiting room areas and a patient information leaflet that should be given to each patient at an appropriate time.
- In England, since October 2022, QOMS is also subject to the National Data Opt-out (NDO). Patients who are registered to the NDO registry should not have their data entered in QOMS. Participating teams should contact their Information Governance department to see what the process is to implement the NDO.
- A patient can contact their treating team or the QOMS Team to object their data being used for the project (regardless of NDO).
Which units are taking part in QOMS?
QOMS is open to every unit in the UK. The following units are already taking part:
Last updated: February 2024